Deep Dives: What is the Difference Between “Exempt” Human Subjects Research, and Projects that are Not Human Subjects Research (NHSR)?
Many areas of the regulations for human subjects research are incredibly dense and nuanced. This “Deep Dives” blog post series is intended to delve into these thorny regulatory questions. View all the posts in this series by visiting the “deep dives” tag on our blog. This post focuses on the difference between “exempt” human subjects research and projects which are considered “Not Human Subjects Research” (NHSR).
The Human Subjects Research (HSR) Equation
In order for a project to be subject to IRB review, it must meet the definition of “Human Subjects Research” (HSR). HSR can be broken down into two component parts: “research” and “human subjects.” Each of these terms have specific regulatory definitions. A project must meet BOTH definitions in order to be considered HSR, and therefore subject to the regulations, including IRB review.
The image below illustrates the concept of HSR: picture a venn diagram, where in the circle on the left we have “research,” and in the circle on the right we have “human subjects.” In the overlap, we have “Human Subjects Research – IRB Review Required.”
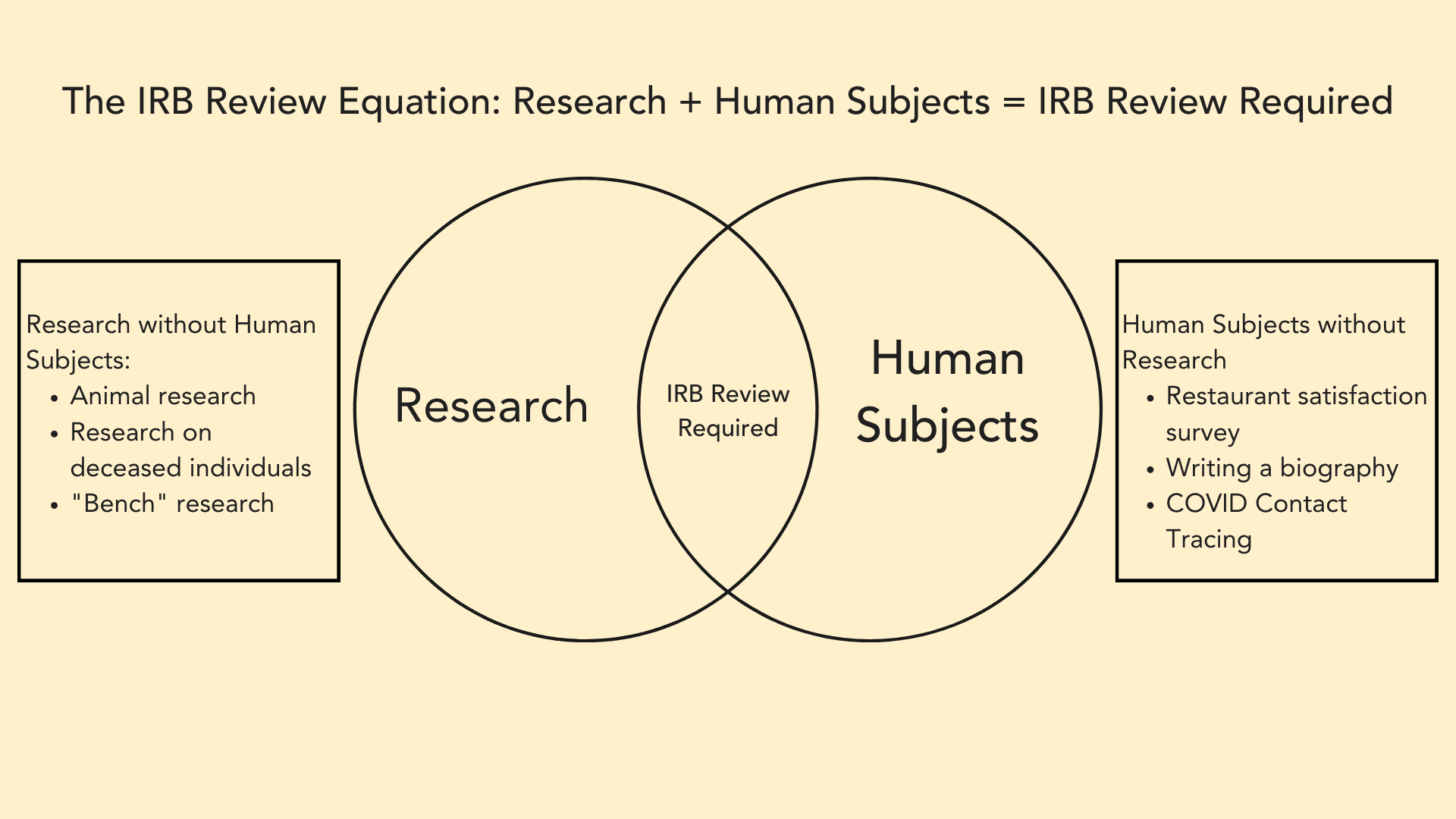
On the left side of the diagram, we have projects that are research, but which don’t involve human subjects. For example, research with animal subjects, or research on deceased individuals, such as certain anthropology research. These projects do not require IRB review.
On the right side of the diagram, we have projects that involve human subjects, but which aren’t research. For example, a satisfaction survey a restaurant asks you to complete, or writing a biography on a specific individual. These projects also do not require IRB review.
Only those projects which meet BOTH regulatory definitions of “research” and “human subjects” are subject to IRB review.
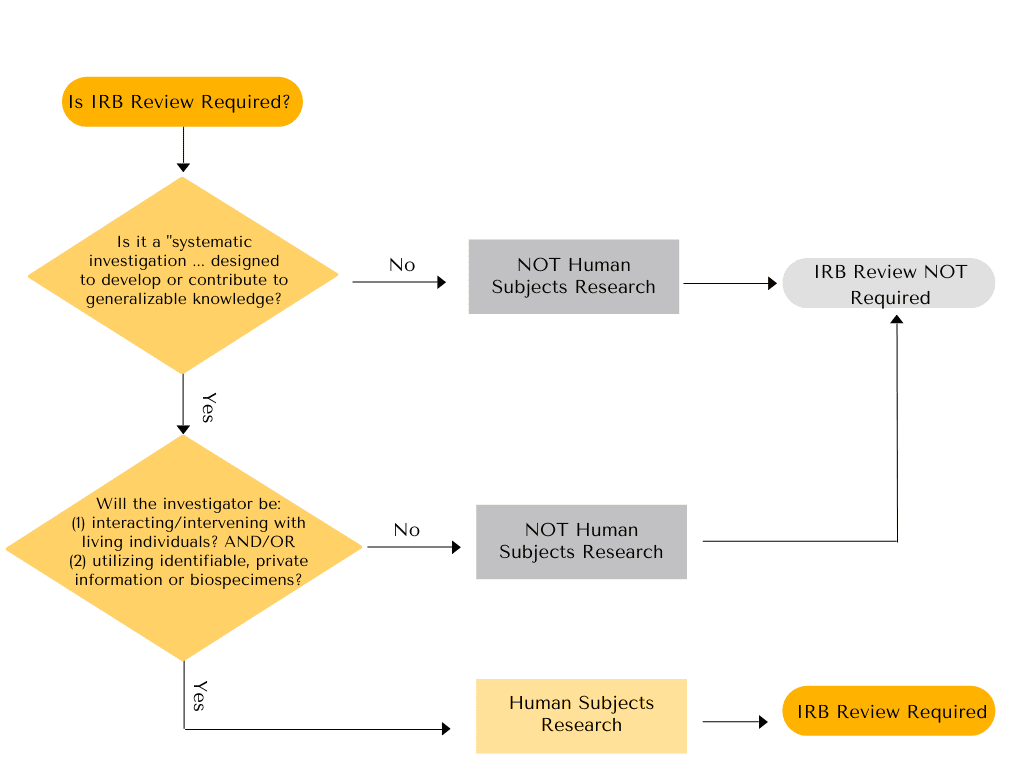
What is (Human Subjects) “Research”?
The first step in determining whether a project is HSR that requires IRB review or whether it is Not Human Subjects Research (NHSR) is to determine if a project meets the definition of “research” according to the HSR regulations. Projects which meet the definition of “research” within the HSR regulations are considered to be human subjects research. Projects which do not meet the definition of “research” according to the HSR regulations may still be considered “research” in other contexts, but these projects are not human subjects research, under the HSR regulations.
Research is defined in the HSR regulations (45 CFR 46.102(l)) as:
“A systematic investigation, including research development, testing, and evaluation, designed to develop or contribute to generalizable knowledge.”
“Generalizable Knowledge” – A Clarification for Social/Behavioral/Educational (SBE) and Qualitative Research
The HSR regulations were written mainly with biomedical research in mind. The VCU HRPP recognizes that the term “generalizable knowledge” is not necessarily the most appropriate term when referring to social/behavioral and educational (SBE) research, particularly research utilizing qualitative methodologies.
However, while these research studies may not be strictly “generalizable” (a more appropriate term might include “transferrable”), these studies are often still seeking to contribute to an overall body or field of knowledge. In this way, the VCU HRPP believes that the spirit of the regulations still applies to these kinds of research projects.
So, when SBE/qualitative researchers consider whether their project meets the definition of (human subjects) “research,” they should consider the intentionality of their project: are you trying to add to, refine, or refute existing knowledge? Are you attempting to demonstrate the effectiveness of an intervention/process outside of your specific context and institution? Are you trying to make broad claims regarding a certain population or phenomenon? If the answer to any of these questions is yes, then your project is likely to meet the definition of (human subjects) “research” under the HSR regulations.
Exceptions to the Rule: When is Research Not (Human Subjects) Research?
The HSR regulations include specific exceptions to the definition of (human subjects) “research.” These are specific kinds of activities that often include human subjects, but which the HSR regulations specifically consider to NOT be (human subjects) research. These kinds of projects are therefore not required to undergo IRB review. Each of these exceptions is discussed below.
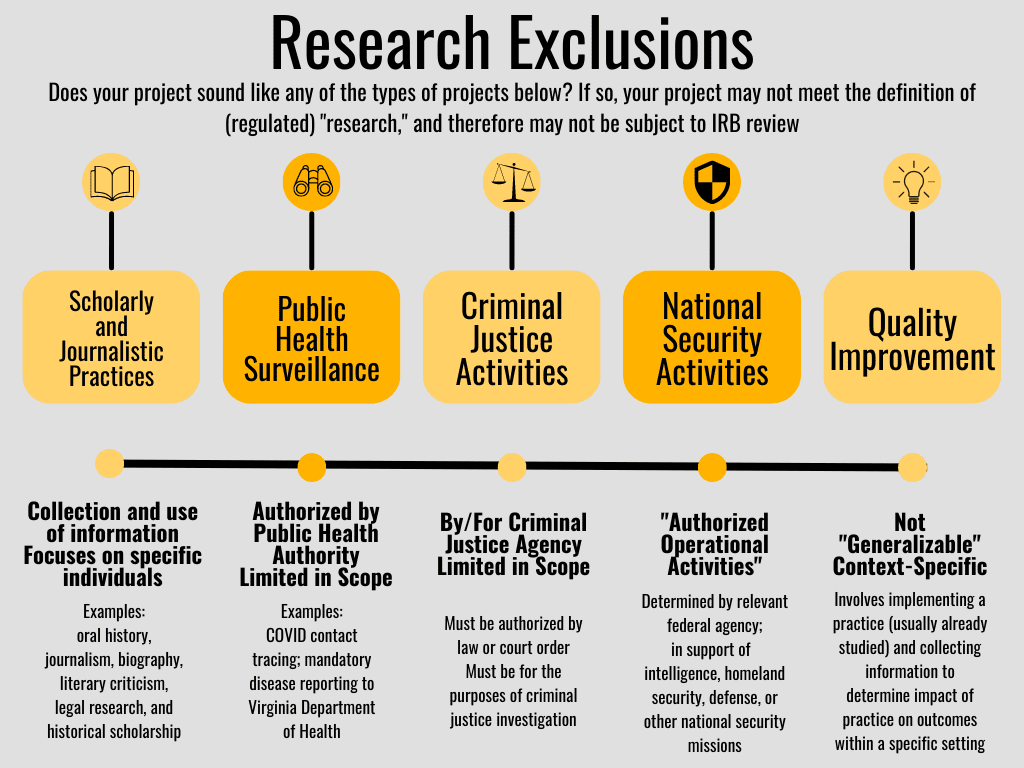
Scholarly and Journalistic Activities
This exclusion for scholarly and journalistic activities includes activities such as oral history, journalism, biography, literary criticism, legal research, and historical scholarship. These are activities that involve the collection and use of information, but which focus directly on the specific individuals about whom the information is collected. For example, writing a biography of a living person would not be considered (human subjects) research under the HSR regulations and would not be subject to IRB review, because such an activity meets the exclusion category for “scholarly and journalistic activities.”
Public Health Surveillance Activities
This exclusion is for activities conducted, supported, requested, ordered, required, or authorized by a public health authority. A “public health authority” is defined as “an agency or authority of the United States, a state, a territory, a political subdivision of a state or territory, an Indian tribe, or a foreign government … that is responsible for public health matters as part of its official mandate.” For example, the CDC or the Virginia Department of Health would be considered “public health authorities.”
This exclusion applies only to activities that are limited to “those necessary to allow a public health authority to identify, monitor, assess, or investigate potential public health signals, onsets of disease outbreaks, or conditions of public health importance … Such activities include those associated with providing timely situational awareness and priority setting during the course of an event or crisis that threatens public health (including natural or man-made disasters).”
So, in order to fall under this exception, a project must be limited in scope to identify, monitor, assess, or investigate public health concerns, AND must be specifically authorized by a public health authority.
A great example of this exception in action is COVID contact tracing. COVID contact tracing is often initiated and conducted by public health authorities, and is limited in scope to tracking and managing the spread of the disease. These projects do not require IRB review, as they fall under this specific exception to the definition of (human subjects) research.
Criminal Justice Activities
This exception refers exclusively to activities performed by or for a criminal justice agency for activities authorized by law or court order solely for criminal justice or criminal investigative purposes. So, for example, collecting DNA samples from suspects in a criminal case, for the purposes of DNA testing within the context of a criminal justice investigation. These activities are excluded from the definition of (human subjects) research under the HSR regulations, and therefore are not subject to IRB review.
National Security Activities
This exception refers exclusively to authorized operational activities (as determined by each agency) in support of intelligence, homeland security, defense, or other national security missions.
Quality Improvement/Assessment/Assurance Activities
Quality Improvement (also called Quality Assessment, Quality Assurance, Program Evaluation, etc.) activities are not addressed specifically in the text of the regulations along with the other exceptions from the definition of (human subjects) research in the HSR regulations. However, Quality Improvement (QI) activities generally do not meet the definition of (human subjects) “research,” due to the fact that QI activities are often not generalizable.
A whole Deep Dive could be dedicated to QI alone, so this post will not get into too much detail on this topic. Just be aware that the Office of Human Research Protections (OHRP) offers guidance on this topic. VCU also addresses the question of QI vs. Research in this guidance document.
What is “Not Human Subjects Research” (NHSR)?
Projects that are considered “Not Human Subjects Research” (NHSR) are projects which are not subject to IRB review, usually because they do not meet the definition of (human subjects) research according to the HSR regulations, and occasionally because they do not meet the definition of “human subjects.”
Regardless of the reason why the definition of “human subjects research” is not met, NHSR projects are projects that are not subject to IRB review. IRB review is not required because the project does not meet the definition of (human subjects) “research” (and/or does not meet the definition of “human subject”).
What Does it Mean for a Project to be “Exempt”? What is the Difference Between NHSR and “Exempt”?
So what is the difference between projects that are “exempt” versus projects that are NHSR? The key is in understanding what “exempt” means in the context of the HSR regulations.
What Does it Mean for a Project to be “Exempt”?
IRB review is divided into three levels of review, and the level of oversight a project receives from the IRB is directly proportional to the level of risk involved in a project. Consider the image below for an illustration of this point:
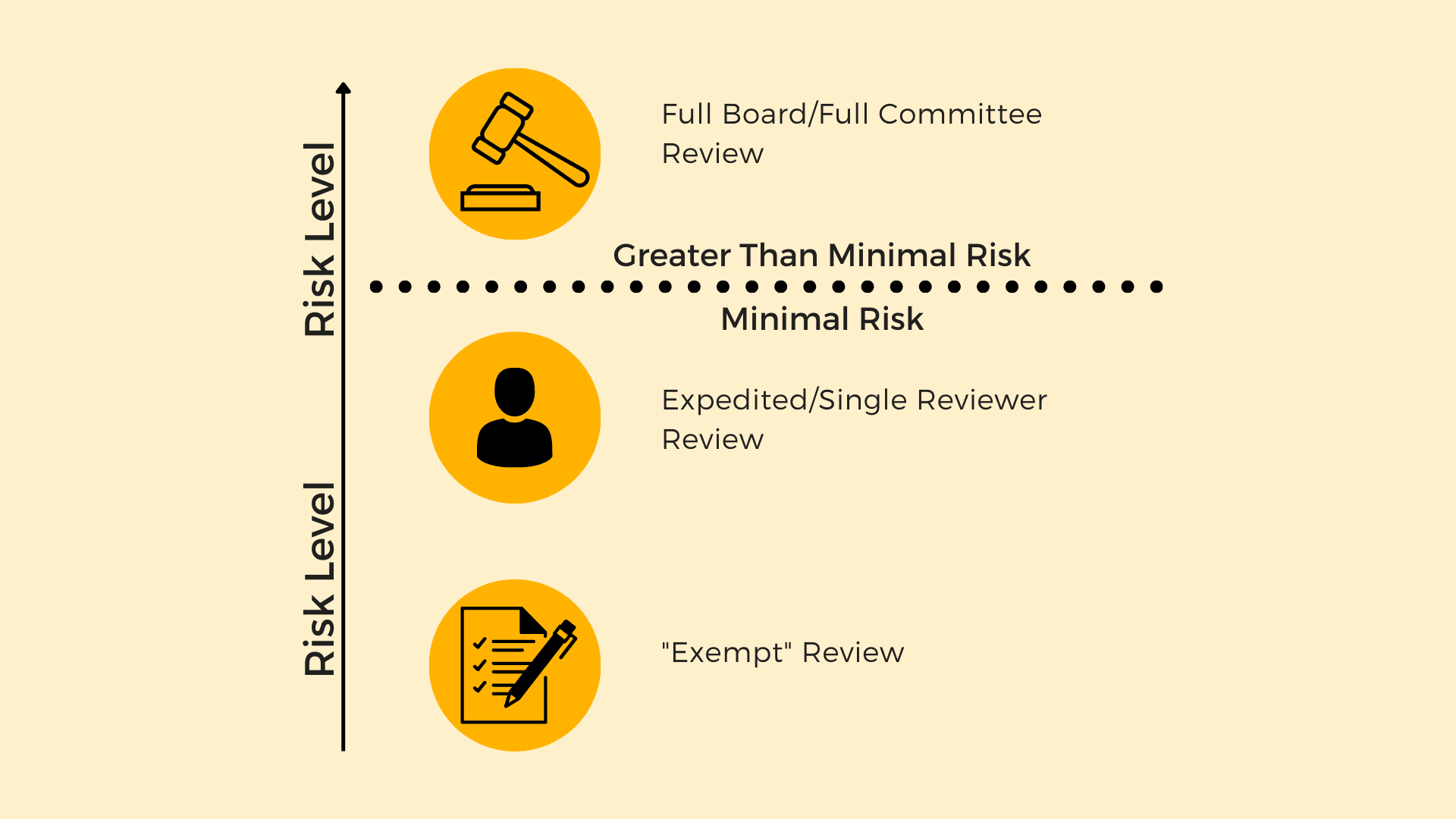
Imagine a continuum where risk increases as you move from bottom to top. At the bottom of the spectrum, there is “exempt” review. Exempt review is reserved for the least risky studies, and receives the least amount of oversight. In the middle, there is “expedited” or “single reviewer” review. This review level is reserved for moderately risky studies, and receives a moderate level of oversight. At the top of the spectrum is full board or full committee review. This level of review is reserved for the riskiest studies, and provides the greatest amount of oversight. The main distinction between full board/full committee review and the other two, lower levels of review is that exempt and expedited/single reviewer review is allowed only for projects that are “minimal risk.” Full board/full committee review is used for projects that are greater than minimal risk.
So what exactly does “exempt” mean in this context? Technically, the “exempt” in “exempt review” means “exempt from the regulations.” Exempt research represents classes of research activities that we have agreed are low enough risk that the full set of regulations do not need to be applied to those projects. So, technically, institutions are not required to apply the regulations to exempt research projects, meaning that IRB review is not strictly required for exempt research projects.
However, institutions can, and often do, implement requirements that go above and beyond the regulations. These institutional requirements often include a requirement to submit exempt research projects for some kind of IRB review and approval. VCU is one such institution. At VCU investigators may not conduct exempt research until the project has been reviewed and approved by the IRB.
The Difference: Exempt vs NHSR
On a strictly technical level, the key difference between exempt HSR and NHSR is that NHSR projects are projects that are NOT human subjects research, and therefore do not require IRB review. Exempt projects, on the other hand, are projects that ARE human subjects research, but are exempt from the regulations, including the requirement for IRB review.
So the key is that NHSR does not require IRB review because the project is not human subjects research at all. Whereas exempt research projects ARE human subjects research, but may or may not be subject to the requirement for IRB review. This is because, while they ARE human subjects research, the risk level of the research is low enough that we have agreed that the research project can be exempt from the regulations, including the requirement for IRB review.
HOWEVER, it is crucial to understand that VCU does require IRB review for exempt research. There are a few reasons for this, including the fact that the VCU HRPP is accredited by the Association for the Accreditation of Human Research Protection Programs (AAHRPP), which requires accredited institutions to implement procedures for reviewing and approving exempt research. In addition, the regulations require, for some types of exempt research, a “Limited IRB Review.” Limited IRB review consists of verifying certain standards are met for exempt research that collects identifiable, sensitive information. Such standards include adequate privacy and confidentiality protections. The VCU IRB achieves the Limited IRB review requirement through its process for reviewing and approving exempt research.
What are the Different Processes for NHSR Projects vs. Exempt HSR?
Since NHSR projects do not require IRB review, investigators can conduct NHSR projects without consulting the IRB. However, the VCU IRB offers a special submission type where investigators can request an official NHSR determination letter from the IRB, which states that the project is NHSR, and therefore not subject to IRB review requirements.
Submitting for an official NHSR determination is not required by VCU. However, it may be desirable to obtain an official determination, as many journals and conferences request such documentation along with submitted presentations/articles. You can learn more about how to submit an NHSR request in this NHSR blog post.
Investigators who are conducting exempt research must use the usual submission process for all HSR projects, using the electronic submission system, RAMS-IRB. You can learn more about how to use RAMS-IRB, including submitting a new study, by browsing the “researcher gif guides” tag on our blog. This tag aggregates all the “Gif Guides” posts. Gif Guides are blog posts that use narrative descriptions and animated .gif images to illustrate how to navigate RAMS-IRB.
Resources for Distinguishing Between NHSR and Exempt HSR
The VCU HRPP offers resources to assist investigators in determining if their project is NHSR, exempt, or some other level of IRB review.
VCU Resources:
- Activities requiring IRB review webpage
- Review types and requirements webpage
- QI vs. Research Resources
- NHSR submission process explanation blog post
OHRP Resources:
- Human research decision trees
- Charts 02-08 assist in determining exemption and exempt category(ies)
- Quality Improvement FAQ Guidance
In addition, the following infographics/flowcharts may be helpful to investigators seeking to determine if their project is NHSR or if it is exempt HSR.
Categories Education and Training