Fall 2019 Patch: Updates to Personnel and the Future Sharing Page
On Friday, October 4th, a patch will be made to the RAMS-IRB system to make updates to the smartform that will impact investigators and research staff. The two main changes to be made in the patch relate to Personnel Updates and the Future Sharing page. The changes made to these pages are described below.
We welcome your feedback on these changes and their implementation! Both of these changes have been driven by the feedback of our research community, and we rely on your continued feedback to further refine and improve our processes and RAMS-IRB. If you have constructive feedback regarding the changes implemented in this patch, please send an email to [email protected] with “Smartform Suggestions” in the subject line.
Changes to Personnel Updates:
On August 1, 2019, ORSP changed its policy relating to updating study personnel in RAMS-IRB. Following this policy update, only changes in Key Personnel are required to be submitted and approved by the IRB in an amendment. Changes in Non-Key Personnel do not require IRB approval.
The patch includes changes in the smartform to reflect and operationalize this policy change. For example, the wording and instructions on the Personnel Page within the smartform have been updated to reflect that only Key Personnel should be listed.
After the patch, Non-Key Personnel may be updated from the main study workspace using the “Study Roster” Activity under the My Activities menu.
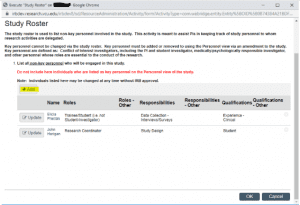
Clicking the “Study Roster” button will open a pop-out window where Non-Key Personnel may be added by clicking the “Add” button at the top of the roster.
Clicking “Add” will open another pop-out window, where you will enter the individual’s name, confirm they are not Key Personnel, and indicate their role, qualifications, and the individual’s start date on the project.
Updates to Key Personnel will be completed through submitting an amendment to the study in RAMS-IRB.
As a reminder, Key Personnel are defined as: Conflict of Interest (COI) investigators, including the PI and student/trainee investigator, medically/psychologically responsible investigator; and other personnel whose roles are essential to the conduct of the research.
Note: Non-Key Personnel are not required to be listed in the roster in RAMS-IRB. However, PIs bear the responsibility to document the delegation of responsibilities. The maintenance of a delegation log or study staff roster may be required by other regulations, sponsor requirements, etc. PIs may elect to use the study roster in RAMS-IRB as a means to document study staff and delegation of responsibilities, but this is not required by VCU policy.
For questions relating to this policy change, please contact [email protected]. If you are unsure whether an individual is Key Personnel or not, you are encouraged to reach out to your assigned IRB coordinator for guidance.
Changes to the Future Sharing Plan Page:
The changes to the Future Sharing Plan page are driven by the feedback provided by investigators, research staff, IRB members and IRB staff. The purpose of the Sharing Plan page is threefold:
- This page creates a plan for when and how information/specimens are shared or used outside of the current protocol.
- This page demonstrates the ethical principle of Respect for Persons by confirming that plans for sharing do not go against what participants would understand about the use of their data/specimens in the consent form.
- The page also ensures there are adequate protections for the privacy of participants and the confidentiality of data/specimens when they are shared with others.
The new Sharing Plan page will use checkbox options rather than free-text responses to help investigators determine the method of sharing and the format of the data/specimens to be shared.
NOTE: The questions on the page now differentiate between 1) research use by the same investigator/research team, and 2) use by individuals or groups outside of the research team.
Question #3 on the Sharing Plan page will ask investigators to indicate the way(s) that individual-level information or biospecimens (including DNA) may be used by the VCU PI or VCU study team for other future research projects (i.e. analyses beyond/apart from the aims of the study described in the smartform).
Question #4 on the Sharing Plan page will ask investigators to indicate the way(s) the VCU PI/study team may share individual-level information or biospecimens (including DNA) with other researchers who are not on the study team of the study described in the smartform (i.e. for analyses beyond/apart from the aims of the study described in the smartform).
The response options for each question are similar, and new questions or smartform pages may appear based on the selections made in each question. The possible response options are described and defined in the table below. This table also describes the branching questions/pages that will appear when the selection is made. As a reminder, the Help text within the smartform can be accessed by clicking the blue question mark icon that follows the text of the question. ![]() The definitions of all the terms used on this page can be found in the Help text.
The definitions of all the terms used on this page can be found in the Help text.
| Option | Description | Definition | Branching Logic |
| 1 | Will use/share directly identifiable information/specimens. | Directly identifiable means that identifiers (i.e.: name, medical record number, social security number, etc.) are included in/attached to the dataset/specimens. | Causes the Creating New Registry/Repository page to appear. Please see below for notes on registries/repositories. |
| 2 | Will use/share de-identified or indirectly identifiable information/ specimens | De-identified means that a linkage/key code exists that links identifiers (i.e.: name, medical record number, social security number, etc.) to data/specimens. When an investigator has access to both the dataset/specimens and the linkage/key code, VCU considers the data/specimens to be readily identifiable. | Causes the Creating New Registry/Repository page to appear. Please see below for notes on registries/repositories. |
| 3 | Will use/share anonymized information/specimens. | Anonymized means that (1) no linkage/key code exists that links identifiers to data/specimens, and (2) subjects cannot be readily identified through the use of indirect identifiers (i.e.: dates [such as date of birth], demographic information, etc.) and/or through combinations of the remaining data. | Will cause two new questions to appear on the Sharing Plan page: (1) to select options to describe how future use of data was covered in the consent form; (2) to certify that resulting dataset contains no HIPAA identifiers and cannot be used to re-identify subjects. |
| 4 | Will use/share aggregate results, not individual-level information/specimens | Aggregate data are summary results from a study. Since data are not individual-level when aggregated, they are not considered to meet the definition of “human subject.” Any investigator receiving aggregate data from another investigator should check with their IRB regarding review requirements. | None. |
| 5 | Will contribute to an existing registry or repository | Data/specimens from the current project will be included in a registry/repository already in existence, either at VCU or outside of VCU. | Causes the Existing Registries/Repositories page to appear. Please see below for notes on registries/repositories. |
| 6 | Will submit data to an NIH genomic data repository | NIH mandates sharing of genomic data from certain studies that analyze genetic information. This type of sharing requires an institutional certification process. | Causes the Genomic Data Sharing page to appear. |
| 7 | Will not use information/specimens for purposes beyond this study/Will not share information/specimens with other researchers | To be used when no future use by the VCU study team and no sharing to those outside the study team is intended. | None. |
| 8 | Not sure and will submit an amendment when known | To be used when future uses/sharing of data/specimens is unknown at the time of submission. | None. Investigators are expected to submit an amendment to describe their future use/sharing plans when they are known. |
| 9 | Other uses/sharing of individual-level information not described in the other options | To be used when the above options do not describe the intended future use/sharing. | Causes a new question to appear on the Sharing Plan page to capture information on the proposed future use/sharing. |
Notes on Registries/Repositories: A registry/repository is defined as a collection of identifiable data/specimens maintained for future, unspecified research. Maintaining directly identifiable or de-identified data/specimens for research outside the aims of the study under which the data/specimens were originally collected qualifies as a registry/repository.
When an investigator uses their own directly identifiable or de-identified data/specimens for research outside the aims of the original study, this use is always considered to be human subjects research requiring IRB review. When an investigator shares de-identified data/specimens with a different investigator for research outside the aims of the original study, the receiving investigator’s use of the de-identified data/specimens may or may not constitute human subjects research, depending on the circumstances. The receiving investigator is responsible for ensuring their activities and use of the data/specimens receive appropriate IRB review, when needed.
Categories Announcements and Updates