Process Improvement and Transformation
What is happening?
Human subjects research at Virginia Commonwealth University (VCU) has grown in complexity and volume over the last five years. Therefore, the VCU Human Research Protections Program (HRPP) is transforming its processes to meet the needs of the institution’s exponential growth. Hence, the VCU HRPP is proud to announce that the HRPP has a partnership with Advarra IRB to support its mission to protect the rights, welfare, and safety of human subjects.
What actions are being taken?
VCU’s HRPP is taking a series of actions to increase IRB review capacity, provide more consistent reviews, and enhance compliance. These actions include:
(1) establishing a partnership with the Advarra IRB as a routine IRB review pathway for externally funded investigator-initiated studies to increase IRB review capacity;
(2) streamlining institutional policies and procedures for IRB review through the implementation of the “Huron Toolkit” to increase VCU IRB review efficiency and compliance; and
(3) implementing a streamlined and updated eIRB system to reduce the administrative burden on both VCU investigators and IRB staff.
The expected result is that timelines associated with the IRB review process will improve.
When will these actions be taken?
The 3 steps outlined above will roll out over the next 9-12 months. However, the first priority is to increase IRB Review Capacity by immediately partnering with the Advarra IRB as a routine IRB review pathway for a subset of investigator-initiated studies.
What does this mean for you as an Investigator at VCU?
The following categories of projects will continue to be reviewed by the VCU IRB or HRPP Office with no changes to the current process:
- Projects requiring a “Not Human Subjects Research” (NHSR) determination
- Projects requiring a determination of exemption
- Emergency Use, Expanded Access, Compassionate Use, HUD, and Single-Patient IND/IDE studies (unless the external sponsor requires use of an external IRB)
- Investigator-Initiated Human Subjects Research projects supported by the department or miscellaneous funds
- Multisite Human Subjects Research projects where the VCU IRB will be serving as the IRB of record
- IND/IDE studies where a VCU investigator holds the IND/IDE
- Student-led Human Subjects Research for Scholarship or Course Credit. Note: The PI must be a VCU faculty member
On April 10, 2023, the following categories of projects requiring IRB review will be submitted to and reviewed by the Advarra IRB, unless the funding source requires review by another specific IRB:
- Externally funded Investigator-Initiated Human Subjects Research projects
- Federally funded Human Subjects Research projects
- Industry or privately funded Human Subjects Research projects
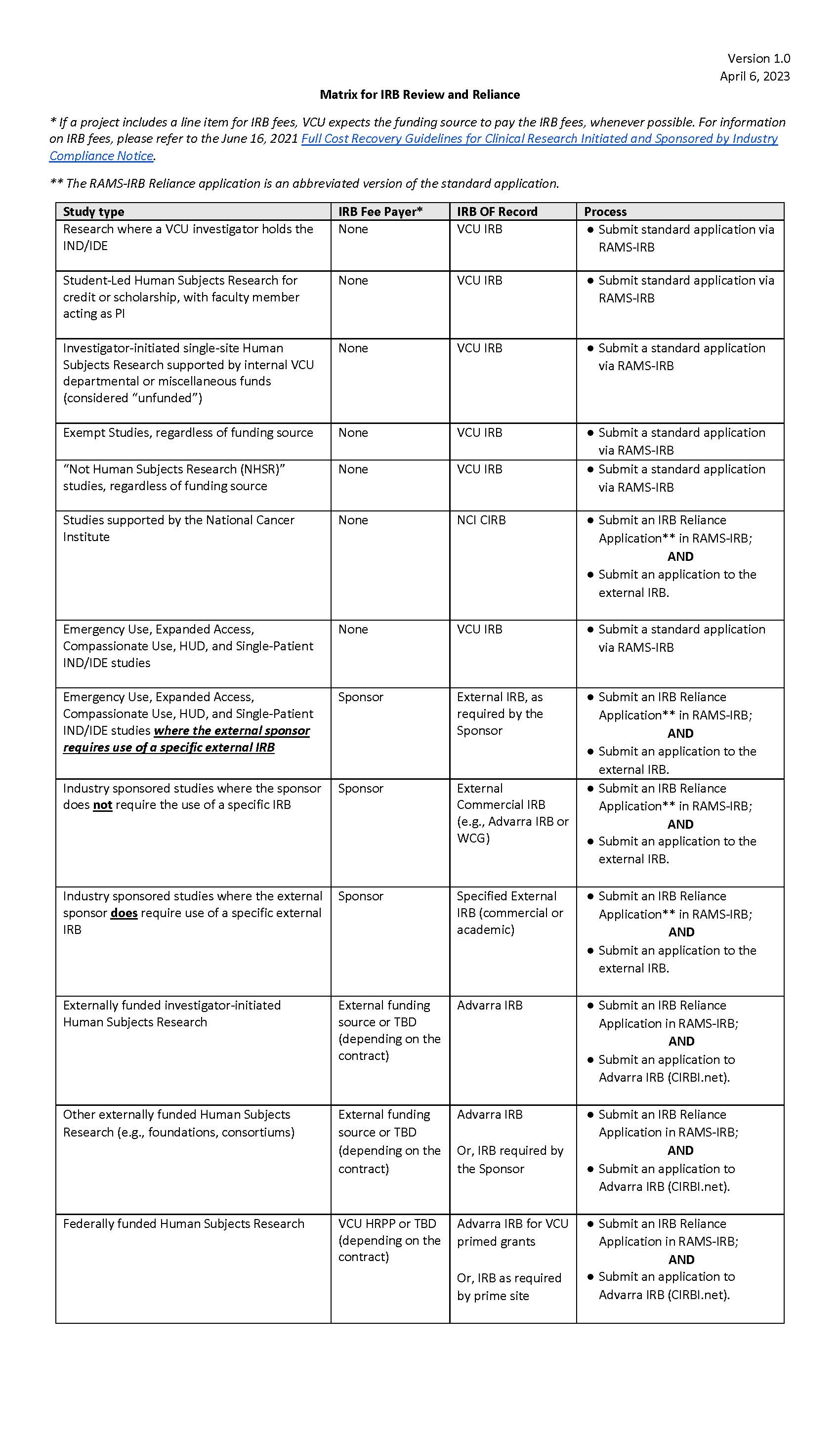
Below is a matrix that outlines which IRB will be the IRB of Record for your future studies. This matrix is for new applications submitted on or after April 10, 2023 only. No changes need to be made to a current RAMS-IRB application, pending or approved, regardless of the IRB of record (i.e., VCU IRB or external IRB).
We foresee that your IRB experience will be much improved as we transform the HRPP. We look forward to better serving you in the near future and beyond as VCU embarks on growing an expansive research portfolio.
Please note: The announcement and/or matrix is subject to change depending on the needs of the VCU IRB and HRPP.
For additional information, don’t hesitate to contact the HRPP Office via [email protected].
Thank you.