ACTION REQUIRED: Human Research Tiering Documentation
EDIT 4/28/2021: As of April 27, 2021, the Tiering system referenced in this post has been discontinued. Investigators are no longer required to make a Tiering determination for their studies.
In order to mitigate the risks of COVID-19 in human research, VCU OVPRI has implemented a Tiering system to stratify research projects based on the potential benefit to participants and the types of interactions involved in the study. This Tiering system provides critical context to the IRB’s review of research, so it is of utmost importance that researchers document the appropriate Tier for each of their studies if they have not already done so.
All Principal Investigators conducting human research at VCU are required to take action to designate the appropriate Tier for each project under their oversight. PIs should review the Tiering system and determine into which Tier each project under their oversight falls, and then must take the appropriate action for that Tier.
Making a Tiering designation and taking appropriate action based on the Tier is required of ALL human research conducted at VCU, regardless of reviewing IRB (i.e.: studies reviewed by WIRB).
Failure to take appropriate action based on the Tier of your research project will result in a delay in IRB approval of the research project.
- Effective immediately, for studies (including initial reviews, continuing reviews, and amendments) submitted for review at the Full Board level, full approval will not be issued until a Tiering designation is on file.
- Beginning 5/16/2020, for studies (including initial reviews, continuing reviews, and amendments) submitted for review at the Exempt or Expedited level, approval will not be issued until a Tiering designation is on file. Studies that are under review prior to that date are strongly encouraged to document their Tiering designation.
PIs can review the COVID-19 and Human Research webpage to learn about each Tier, and to learn about what action is required for each Tier. In brief:
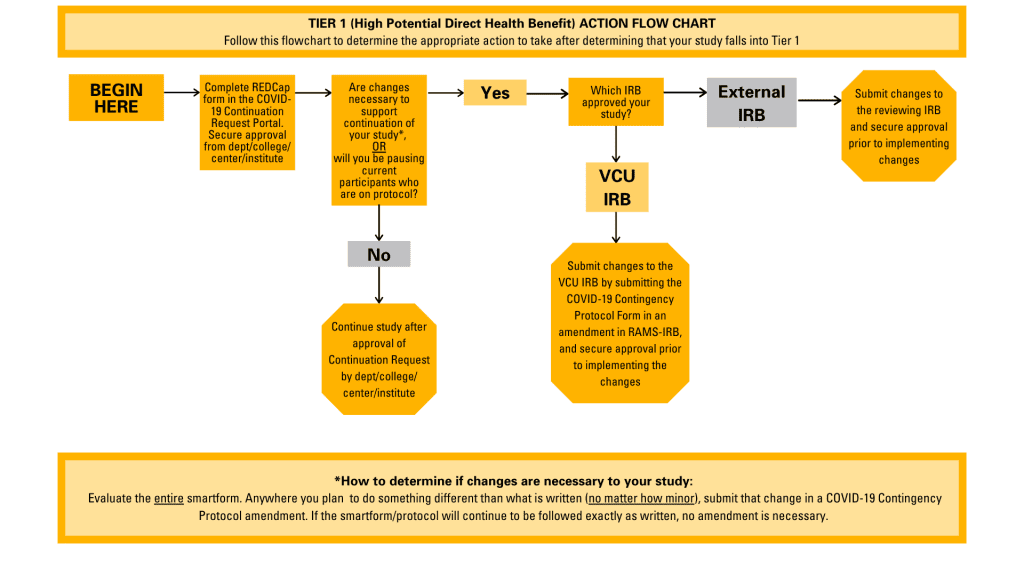
- For Tier 1 AND Tier 2 studies: justification for continuing the study must be submitted through the COVID-19 Human Research Continuation Request Portal for approval by the relevant school/college/department/center. Once approved by the relevant school/college/department/center, a copy of the continuation justification is provided to the IRB, which will serve as documentation of the Tiering designation. If you plan to pause current participants who are on-protocol, an amendment must be submitted to the reviewing IRB. Any changes to support the continuation of the study must also be submitted in an amendment to the reviewing IRB. For studies reviewed by the VCU IRB, PIs must submit an amendment utilizing the COVID-19 Contingency Protocol Form. Instructions for using the COVID-19 Contingency Protocol Form can be found in this blog post. For studies reviewed by an external IRB, amendments must be submitted utilizing the external IRB’s guidance and procedures.
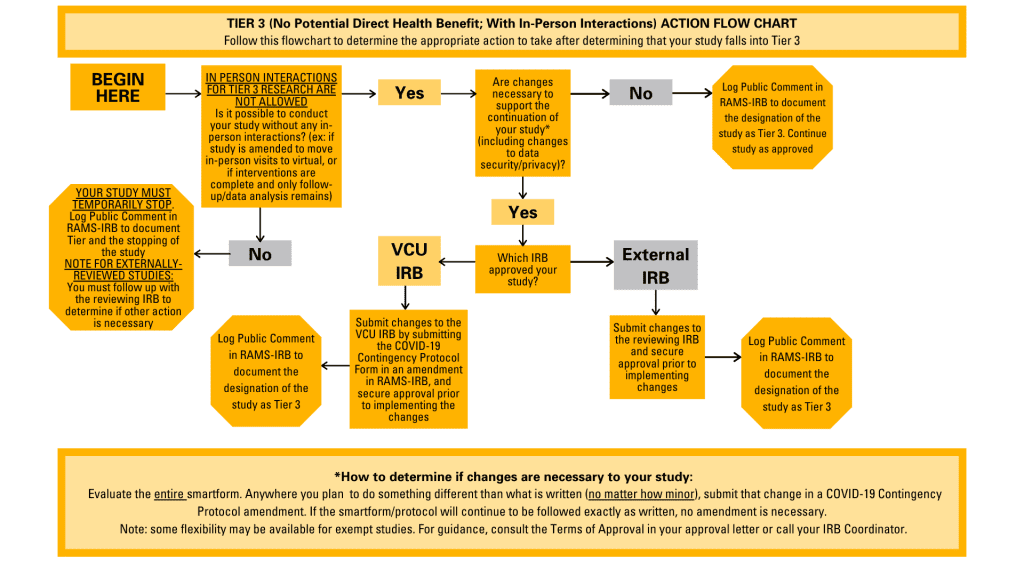
- For Tier 3 studies: PIs must log a Public Comment in RAMS-IRB to document this Tiering designation. If the study will pause some or all of its activities, this should be made clear in the Public Comment. If changes are required to support the continuation of these studies (i.e.: switching in-person activities to be remote), PIs must submit an amendment utilizing the COVID-19 Contingency Protocol Form if the study is reviewed by the VCU IRB. Instructions for using the COVID-19 Contingency Protocol Form can be found in this blog post. For externally-reviewed studies, amendments must be submitted utilizing the guidance and procedures of the reviewing IRB.
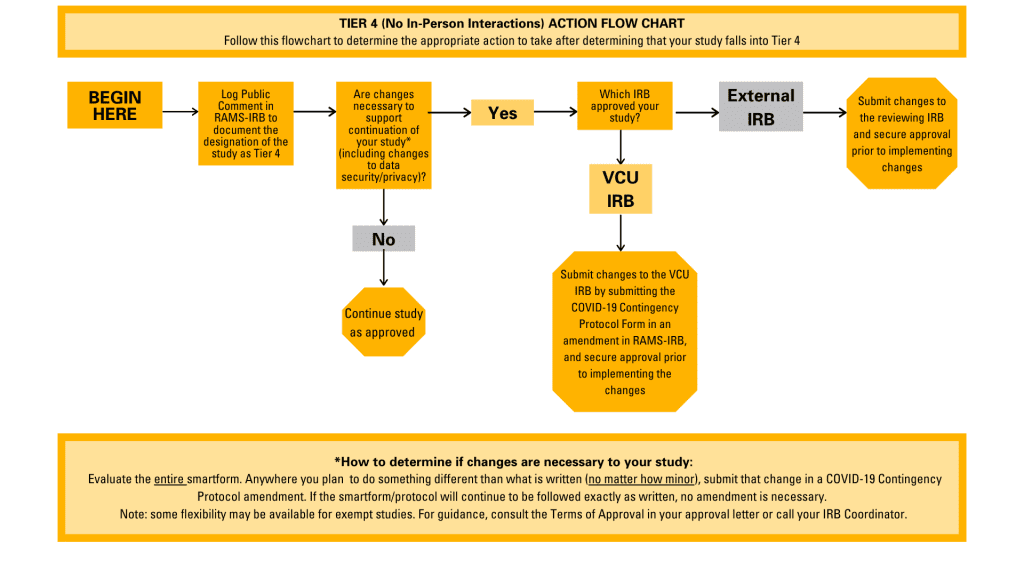
- For Tier 4 studies: PIs must log a Public Comment in RAMS-IRB to document this Tiering designation. If changes are required to support the continuation of these studies (i.e.: changes relating to privacy or confidentiality to support research teams working remotely), PIs must submit an amendment utilizing the COVID-19 Contingency Protocol Form, if the study is reviewed by the VCU IRB. Instructions for using the COVID-19 Contingency Protocol Form can be found in this blog post.
The flowcharts below will assist researchers in determining the appropriate action to take once a study’s Tier has been designated. Click on each image to view a larger version of the flowchart.
The VCU Human Research Protection Program has also developed FAQs that may assist researchers in making Tiering designations and determining the appropriate actions to take based on the Tiering designation. Researchers may also contact their IRB Coordinator for assistance with documenting their Tiering designations, or making changes to a research project to support the continuation of the project.
Questions regarding the Tiering system, the requirements of each Tier, and the consequences of not documenting a Tiering designation may be directed to ORSP@vcu.edu

A flowchart illustrating the appropriate actions to take for Tier 1 studies. 
A flowchart illustrating the appropriate actions to take for Tier 2 studies. 
A flowchart illustrating the appropriate actions to take for Tier 3 studies. 
A flowchart illustrating the appropriate actions to take for Tier 4 studies.